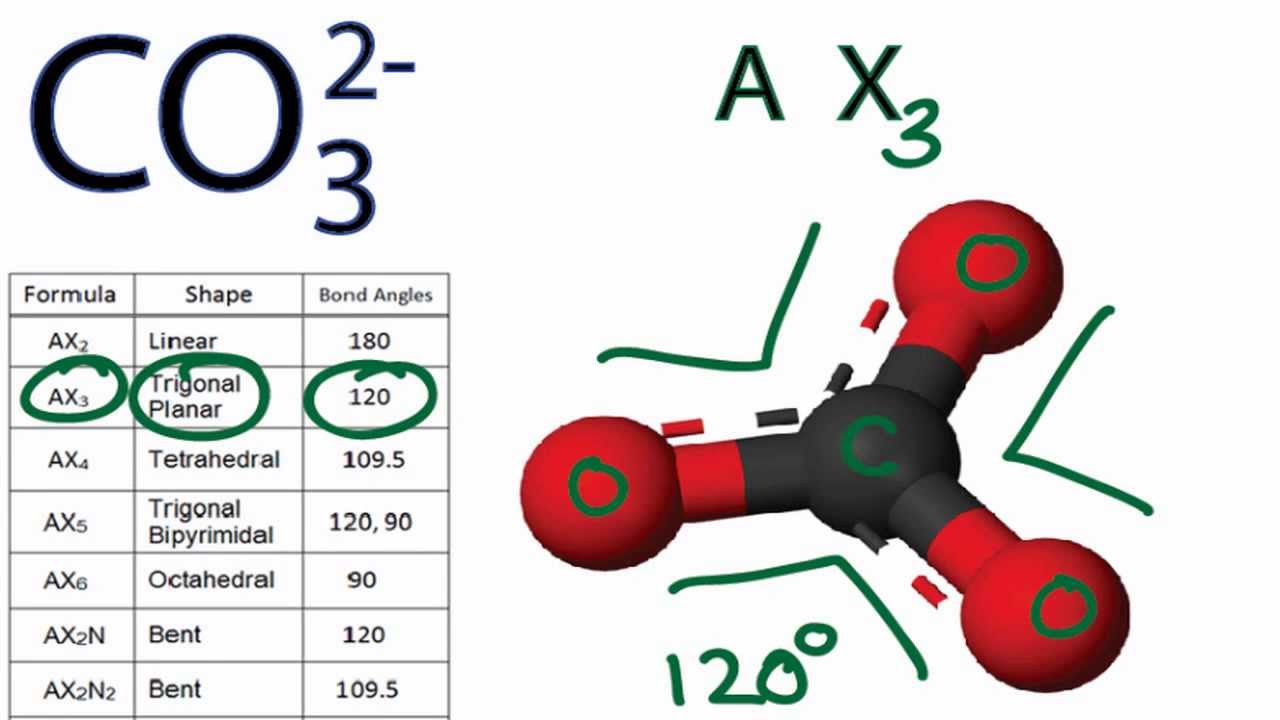
CO3 2 Molecular Geometry / Shape and Bond Angles YouTube
A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion).For the CO3 2- structure use the periodic table to find the total nu.
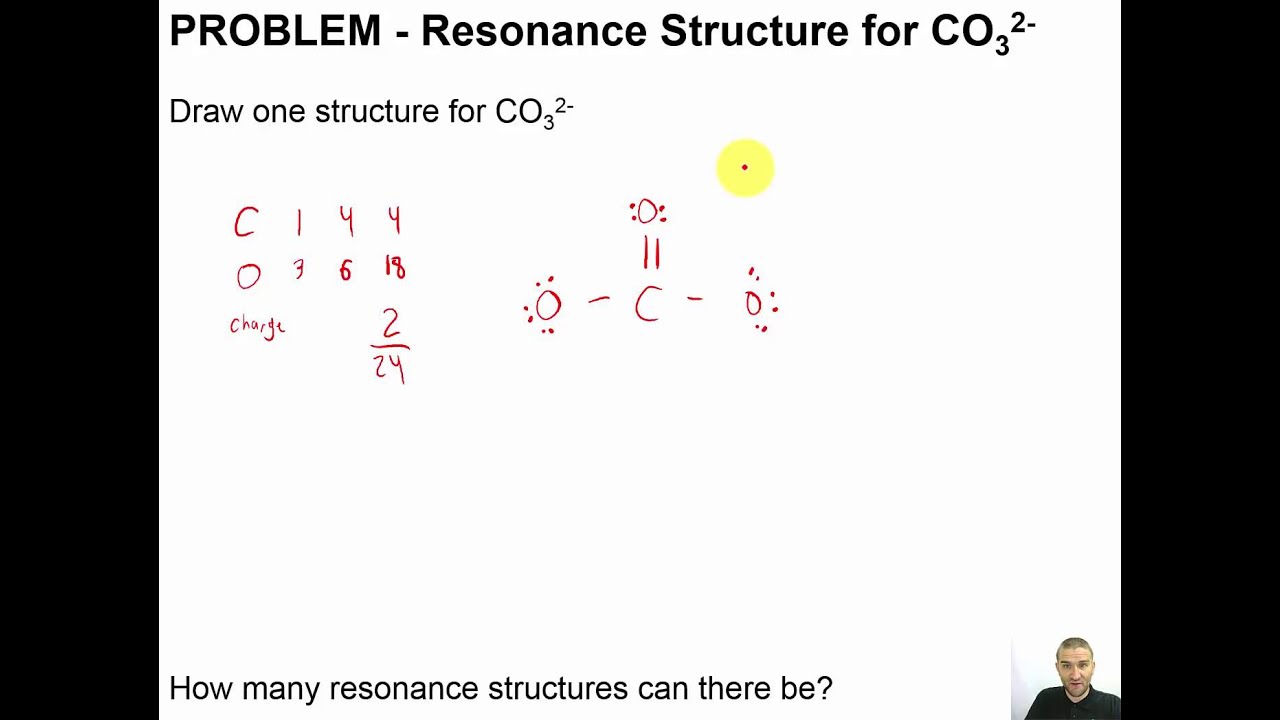
Chapter 05 11 PROBLEM Resonance Structure for CO32 YouTube
A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are measured in Ångstroms (1 Å = 10 -10 m) or picometers (1 pm = 10 -12 m, 100 pm = 1 Å). Figure 5.2.1 5.2. 1: Bond distances (lengths) and angles are shown for the formaldehyde molecule, H2CO.

Hướng dẫn vẽ co3 2 lewis structure đầy đủ và chi tiết nhất
Geometry. CO32- Geometry and Hybridization. There are 4 + 3×6 + 2 = 24 electrons. The carbon goes in the middle, and the oxygens take 6 electrons each as three lone pairs: The carbon lacks an octet, so we use a lone pair from one oxygen to make a double with it. The other two oxygen are then negatively charged:
[Solved] CO32 Electron domain geometry Molecular geometry Approximate
A quick explanation of the molecular geometry of CO3 2- including a description of the CO3 2- bond angles.Looking at the CO3 2- Lewis structure we can see th.

What is the molecular and electron geometry of \ce{CO3^{2} Quizlet
A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion).For the CO3 2- structure use the periodic table to find the total nu.

what is the shape of SO3 , CO3^2, NO3^1plx explain how with Lewis dot
Carbonate is a carbon oxoanion. It is a conjugate base of a hydrogencarbonate. ChEBI. Carbonate Ion is a polyatomic ion with formula of CO3 (2-). NCI Thesaurus (NCIt) Salts or ions of the theoretical carbonic acid, containing the radical CO2 (3-). Carbonates are readily decomposed by acids. The carbonates of the alkali metals are water-soluble.
¿Cuál es la estructura de Lewis de Co3 2?
This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. This video discusses the resonance structu.

Get Molecular Geometry And Angles PNG GM
It has six electrons in valence shell. Total valence electrons given by carbon atom = 4. There are three oxygen atoms in CO 32- ion, Therefore. Total valence electrons given by oxygen atoms = 6 *3 = 18. There are -2 charge on CO 32- ion. Therefore there are two more electrons which contribute to the valence electrons.
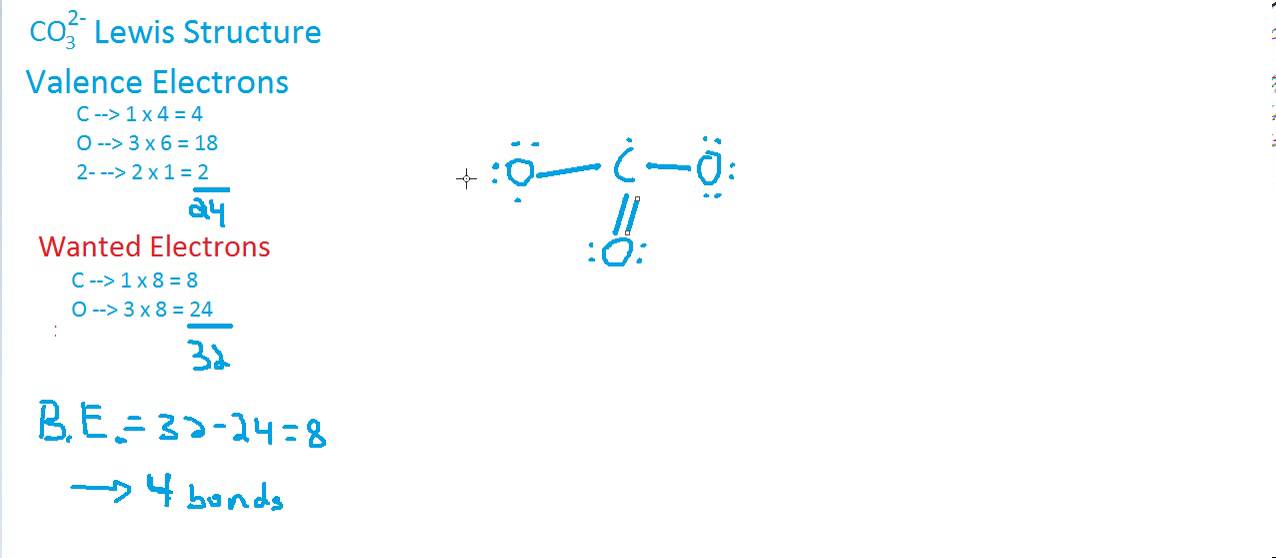
CO3 2 Lewis Structure & Geometry YouTube
Here are the steps to draw the CO3 2- Lewis structure in an active voice: Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. For CO3 (2-), we have 4 valence electrons for carbon and 6 valence electrons for each oxygen, totaling 4 + 6 (3) + 2 = 24 electrons.

탄산이온을 루이스 전자점식으로 나타내는 과정을 알려주세요 지식iN
For the CO 32- Lewis structure there are a total of 24 valence electrons available. Transcript: Let's do the CO3 2- Lewis structure: the carbonate ion. Carbon has 4 valence electrons; Oxygen has six, we have 3 Oxygens, and this negative 2 means we have an extra two valence electrons. Add that all up: 4 plus 18 plus 2: 24 valence electrons.

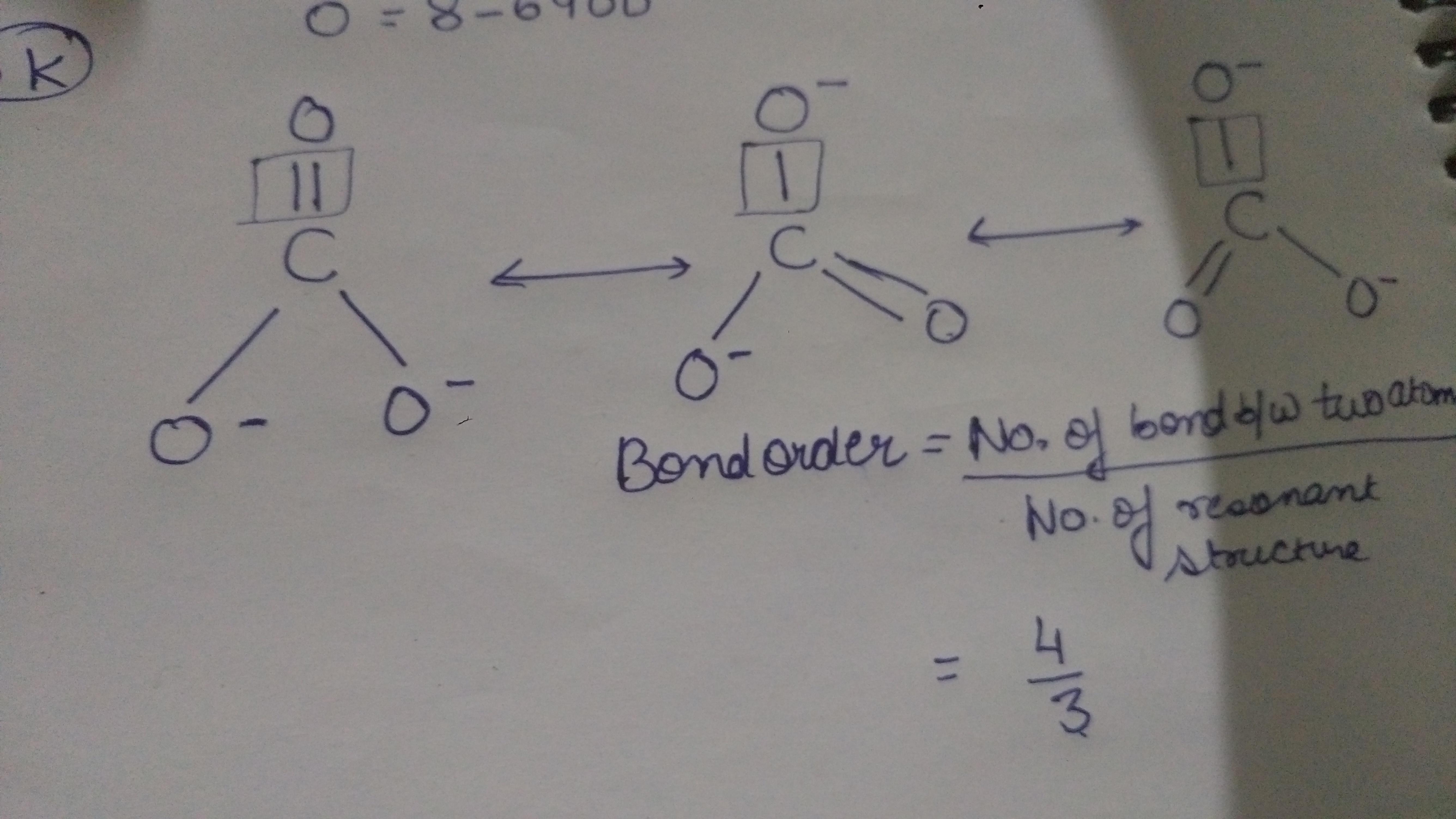
bond Where do the lone pairs go in the "true" resonance structure
Average rating / 5. Vote count: Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry hosted by University of Liverpool.
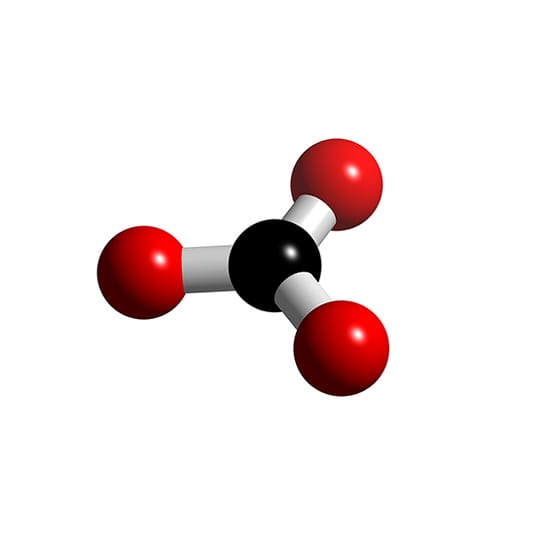
Co3 2 Molecular Geometry
In this comprehensive step-by-step guide, we will explore the Lewis structure of the carbonate ion in detail. We will break down the process into simple and easy-to-follow steps, enabling you to visualize the arrangement of atoms and electrons and grasp the underlying principles governing the chemical bonding within CO3 2-. CO3 2- Lewis Structure Step-by-Step

CO32 Lewis Structure, Molecular Geometry, Hybridization, and MO
Carbonate ion (CO32-) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. CO 32- is the chemical formula for carbonate ion, a polyatomic ion composed of 1 carbon and 3 oxygen atoms. It is present in a carbonate salt i.e., a salt of carbonic acid (H 2 CO 3 ).
Filecaesium Carbonate3dballspng Wikimedia Commons Co3 2 Molecular
CO3 2- Molecular Shape. Finally, let's talk about the overall molecular shape of CO3 2. The molecular shape is the actual three-dimensional shape of the molecule. In the case of CO3 2, the molecular shape is also trigonal planar, since the three oxygen atoms and the central carbon atom all lie in the same plane.

Substance Lewis Structure Zones Molecular Shape Pair Geometry H2O
total valence electron number in CO32- is. = 4 + 6*3 + 2. = 24. Step 2: Determine the Central Atom of the Molecule. Now, in order to draw the Lewis Structure, we have to determine which one is the central atom in a multiatomic heterogeneous molecule, here an ion. In carbonate ion, among the two elements, Carbon has an electronegativity value of.

(Get Answer) 7a. Draw The Lewis Structure Of CO32? 7b. What Is The
Oxidation-Reduction. None. This page titled Carbonate Ion (CO₃²⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Carbonate ion, a moderately strong base, undergoes considerable hydrolysis in aqueous solution. In strongly acidic solution, CO2 gas is evolved.